You are Safe with Bodygard!
Price Offer / Sample RequestYou are Safe with Bodygard!
Price Offer / Sample Request
Products
BODYGARD? SFS LEVEL 3 surgical gowns are available in EN 13795 High Performance/ AAMI LEVEL 3 gowns and suitable for most procedures. BODYGARD surgical gowns SFS65 LEVEL 3 are made from a breathable, textile-like, Superfiber spunbond material that is comfortable to wear.
They are ergonomic too. Shoulder pleats allow freedom of movement for the upper body and the ergonomic sleeves allow comfort and freedom of movement.The gown cuff is compatible with gloves, preventing the gloves from sliding off.
Choose our High Performance gown with impermeable front and sleeves for added protection against strike-through.
Gowns are examples of personal protective equipment used in health care settings. They are used to protect the wearer from the spread of infection or illness if the wearer comes in contact with potentially infectious liquid and solid material. They may also be used to help prevent the gown wearer from transferring microorganisms that could harm vulnerable patients, such as those with weakened immune systems. Gowns are one part of an overall infection-control strategy.
A few of the many terms that have been used to refer to gowns intended for use in health care settings, include surgical gowns, isolation gowns, surgical isolation gowns, nonsurgical gowns, procedural gowns, and operating room gowns.
In 2004, the FDA recognized the consensus standard American National Standards Institute/Association of the Advancement of Medical Instrumentation (ANSI/AAMI) PB70:2003, “Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.” New terminology in the standard describes the barrier protection levels of gowns and other protective apparel intended for use in health care facilities and specifies test methods and performance results necessary to verify and validate that the gown provides the newly defined levels of protection:
Regardless of how the product is named (that is, isolation gown, procedure gown, or cover gown), when choosing gowns, look for product labeling that describes an intended use with the desired level of protection based on the above risk levels. Product names are not standardized.
A surgical gown is regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. A surgical gown is a personal protective garment intended to be worn by health care personnel during surgical procedures to protect both the patient and health care personnel from the transfer of microorganisms, body fluids, and particulate matter. Because of the controlled nature of surgical procedures, critical zones of protection have been described by national standards. As referenced in Figure 1: the critical zones include the front of the body from top of shoulders to knees and the arms from the wrist cuff to above the elbow. Surgical gowns can be used for any risk level (Levels 1-4). All surgical gowns must be labeled as a surgical gown.
Surgical isolation gowns are used when there is a medium to high risk of contamination and a need for larger critical zones than traditional surgical gowns. Surgical isolation gowns, like surgical gowns, are regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. As referenced in Figure 2, all areas of the surgical isolation gown except bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown. Additionally, the fabric of the surgical isolation gown should cover as much of the body as is appropriate for the intended use.
Non-surgical gowns are Class I devices (exempt from premarket review) intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk patient isolation situations. Non-surgical gowns are not worn during surgical procedures, invasive procedures, or when there is a medium to high risk of contamination.
Like surgical isolation gowns, non-surgical gowns should also cover as much of the body as is appropriate to the task. As referenced in Figure 2, all areas of the non-surgical gown except bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown.
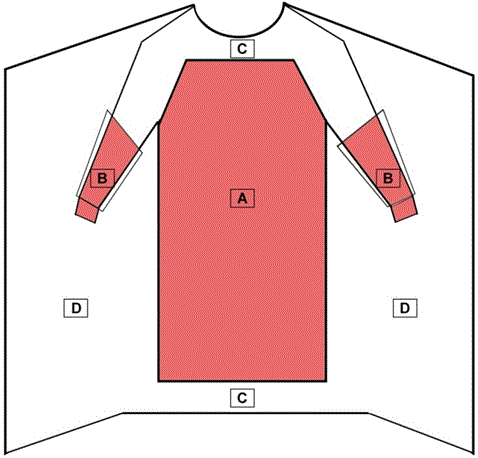
Figure 1 - Critical Zones for Surgical Gowns
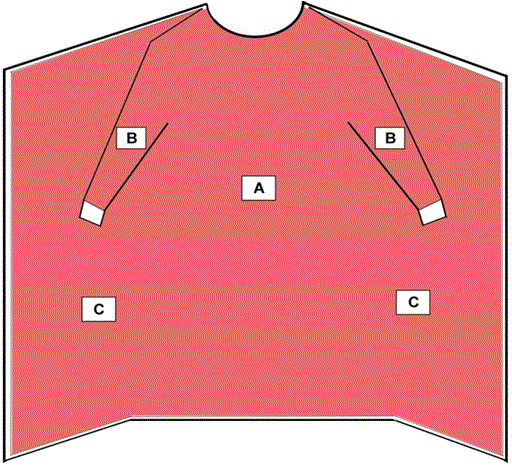
Figure 2 - Critical Zones for Surgical Isolation Gowns and Non-Surgical Gowns
Labeling that shows a product has been tested to and meets appropriate performance standards is one way for users and procurers to determine when to use a particular gown.
The performance of gowns is tested using consensus standards:
American Society for Testing and Materials (ASTM) F2407 is an umbrella document which describes testing for surgical gowns: tear resistance, seam strength, lint generation, evaporative resistance, and water vapor transmission.
Below is a summary of ASTM F2407 standard recognized by the FDA.
American National Standards Institute (ANSI) and the Association of the Advancement of Medical Instrumentation (AAMI): ANSI/AAMI PB70:2003 describes liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.
Below is a table summarizing the ANSI/AAMI PB70 standard recognized by the FDA.
| Type of PPE | Feature Tested | Standard Designation | Sub headings | Description | Applicability |
|---|---|---|---|---|---|
| Gowns | Liquid Barrier Performance | AAMI PB70:2012 | Classifies a gown's ability to act as a barrier to penetration by liquids or liquid-borne pathogens based on four levels. The critical protective zones for surgical and non-surgical gowns are defined differently by the standard. While the critical zones designate different protective areas for the different gowns, the levels of protection are the same for both surgical and non-surgical gowns | Liquid barrier performance is not related to the strength of the material. This standard references several other standards | |
| Level 1 |
| basic care, standard hospital medical unit | |||
| Level 2 |
| Blood draw from a vein, Suturing, Intensive care unit, Pathology lab | |||
| Level 3 |
| Arterial blood draw, Inserting an IV, Emergency Room, Trauma | |||
| Level 4 |
| Pathogen resistance, Infectious diseases (non-airborne), Large amounts of fluid exposure over long periods |
Conformance with recognized consensus standards is voluntary for a medical device manufacturer. A manufacturer may choose to conform to applicable recognized standards or may choose to address relevant issues in another manner.
For a device sold sterile, the FELIX recommends sponsors provide the following information as detailed in the final guidance
Surgical gowns are devices that are considered a surface-contacting device with intact skin with a contact duration of ? 24 hours. The FDA recommends that cytotoxicity (ISO 10993-5), sensitization (ISO 10993-10), and irritation or intracutaneous reactivity (ISO 10993-10) is evaluated for a device. For more information about biocompatibility end point assessment, please refer to the final guidance document entitled, “Use of International Standard ISO 10993-1, “Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process".
Bodygard SFS Surgical Gown LEVEL 3 is now FDA 510K approved. We are honored to be the first FDA 510K awarded surgical gown brand in Turkey. FDA 510K clearance provides added safety and higher quality regulation to our products.
ASTM Level 3 surgical gowns manufactured by Bodygard have recently received 510(k) approval from the US Food and Drug Administration (FDA). Surgical Gowns are designed to be used by healthcare professionals to protect both the patient and themselves from the contamination of microorganisms, body fluids and particulate matter. Surgical Gowns are designed for use in infection control applications to reduce possible exposure to blood and body fluids.
For the US market, Bodygard now provides FDA 510K approved surgical gowns that meet the American Society for Testing and Materials (ASTM) Level 3 standard. PB70 AAMI ,the ASTM Level 3 surgical gown is for use in Moderate water resistance (resistant to water spray and some resistance to water penetration under constant contact with increasing pressure). Masks are a single-use, disposable device supplied non-sterile.
PB70
"Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities"
Description
Overview: This standard establishes a system of classification for protective apparel and drapes used in health care facilities based on their liquid barrier performance and specifies related labeling requirements and standardized test methods for determining compliance.
he ANSI/AAMI PB70 standard includes four standard tests to evaluate the barrier effectiveness of surgical gowns, isolation gowns, and surgical drapes. Based on the results of these standardized tests, four levels of barrier performance are defined, with Level 1 being the lowest level of protection, and Level 4 being the highest level of protection. Table 3 summarizes the requirements of ANSI/AAMI PB70:2012 regarding the classification of barrier performance of surgical gowns, isolation gowns, and surgical drapes.
Table 3: ANSI/AAMI PB 70:12 classification of barrier performance of surgical gowns, other protective apparel, surgical drapes and drape accessories
Level1
| Level1 | Test | Liquid Challenge | Result | Expected Barrier Effectiveness |
| 1 | AATCC 42 Impact Penetration2 | Water | = 4.5 g | Minimal water resistance (some resistance to water spray) |
| 2 | AATCC 42 Impact Penetration | Water | = 1.0 g | Low water resistance (resistant to water spray and some resistance to water penetration under constant contact with increasing pressure) |
| AATCC 127 Hydrostatic Pressure3 | Water | = 20 cm | ||
| 3 | AATCC 42 Impact Penetration | Water | = 1.0 g | Moderate water resistance (resistant to water spray and some resistance to water penetration under constant contact with increasing pressure) |
| AATCC 127 Hydrostatic Pressure | Water | = 50 cm | ||
| 4 | ASTM F1670 Synthetic Blood Penetration Test (for surgical drapes) | Surrogate Blood | no penetration at 2 psi(13.8 kPa) | Blood and viral penetration resistance (2 psi) |
| ASTM F1671 Viral Penetration Test (for surgical and isolation gowns) | Bacteriophage Phi-X174 | no penetration at 2 psi(13.8 kPa) | ||
1 In order of increasing protection 2 American Association of Textile Chemists and Colorists (AATCC) 42 Water resistance: impact penetration test determines the ability of a material to resist water penetration under spray impact [AATCC 2000] 3 AATCC 127 Water resistance: hydrostatic pressure test determines the ability of a material to resist water penetration under constant contact with increasing pressure [AATCC 1998] | ||||
Personal Protective Equipment
Body Protection
All items of clothing and/or accessories (whether or not detachable) designed and manufactured to provide specific protection. This includes bullet-proof clothing, general protective clothing and full body ensembles that protect from cuts, radiation, temperature extremes, hot splashes from molten metals and other hot liquids, potential impacts from tools, machinery and materials and hazardous chemicals. Examples of body protection include laboratory coats, coveralls, vests, jackets, aprons, surgical gowns and full body suits
Bodgard`s surgical Gowns were tested for performance in four areas: impact penetration, hydrostatic pressure, Synthetic blood penetration, and Viral Penetration tets under PB70. Upon completion of testing, the surgical gowns consistently met ASTM Level 3 criteria in all four performance test areas. Synthetic Blood Penetration and Virus penetration test efficiency test results were more than 99 percent.
“This has been a huge success for our team. This is a milestone that supports our commitment to continue to produce high-performance products. Meeting the ASTM Level 3 standard is a stable, reliable way to not only keep our healthcare workers safe but also ensure that famines that occurred at the start of the pandemic don't happen again. It helps us in our goal of ensuring they have a supply of PPE."
Products
Contact
You can reach us by filling out the form below. We will get back to you as soon as possible.
You can sign up for our newsletter to be informed
about innovations about Bodygard.